 Image credit Getty
Image credit Getty
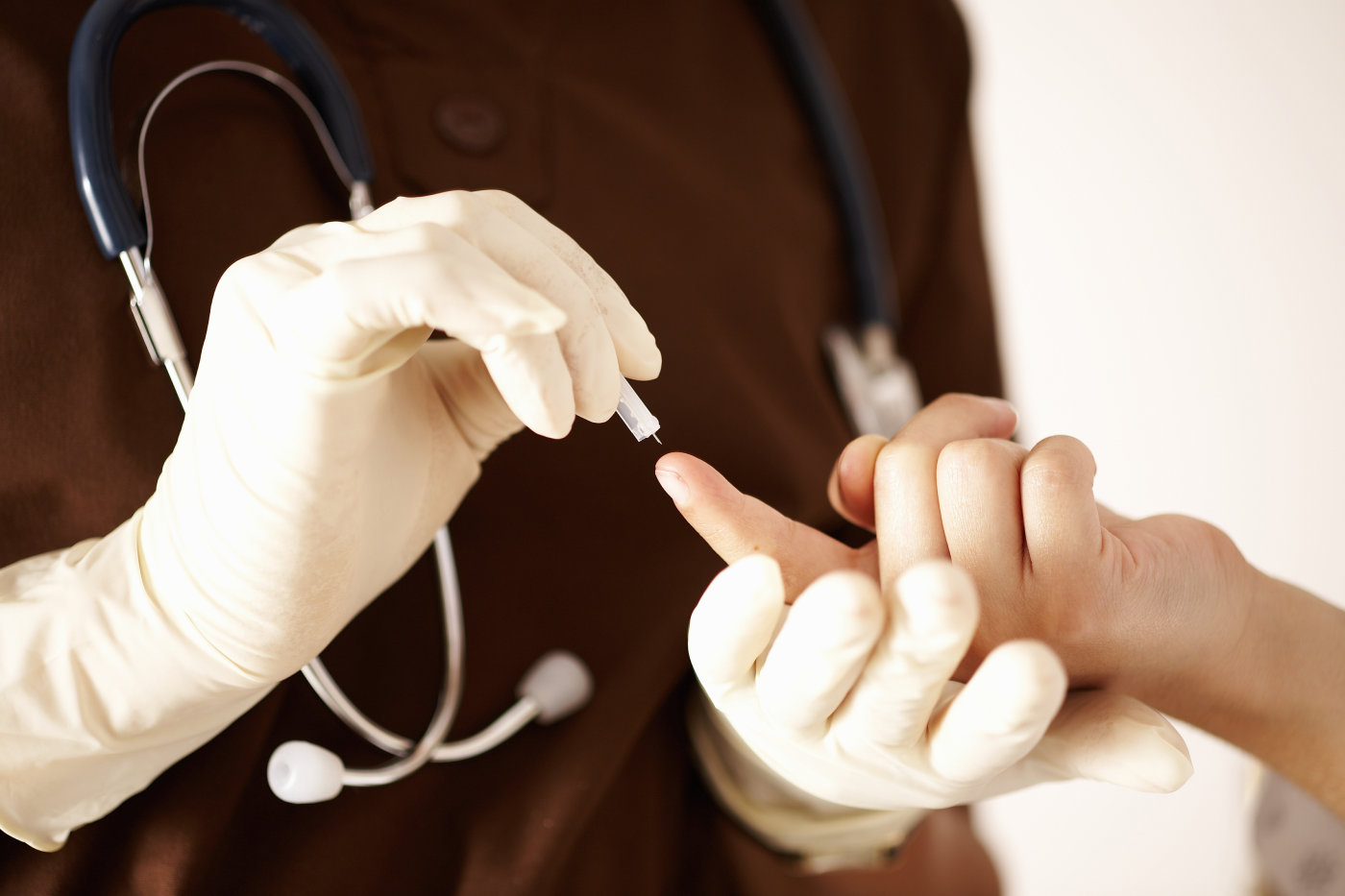
Regulators find big deficiencies at Theranos lab
 Image credit Getty
Image credit Getty
Randall Craig
|
26 January, 2016, 00:02
Recommended
- [TOP STORY] Islamic State video purports to show Paris attackers, threatens Britain
- German business confidence falls more than expected
- Patriots Super Bowl Hopes Dashed in Denver
- Miocic Replaces Velasquez in UFC 196 Main Event
- Saudi Arabia cements support against Iran at Arab League summit
- Toronto cop found guilty of attempted murder in streetcar shooting
- Amy Schumer defends her name after being accused of stealing jokes
- Splinter Taliban group says it carried out Pakistan attack
- Spurs to sit Duncan vs. Warriors
- Amit Shah re-elected unopposed as BJP president
- Malaga snaps up Atsu on loan
- Donald Trump Credits 'Loyalty' For Shooting Comment
- Missing After Collision Of 2 Marine Helicopters Off Hawaii
- Serena Williams wins Australian Open first-round match in straight sets
- Pokemon Super Bowl commercial unveiled, urges us to 'train on'
Trending Now
Dont Miss
Popular destinations
- Best Non Gamstop Casino
- Non Gamstop Sites
- Casino Not On Gamstop
- Casino Not On Gamstop
- Casino Online Non Aams
- Non Gamstop Casinos
- Sites Not On Gamstop
- Non Gamstop Casinos Uk
- Sites Not On Gamstop
- Non Gamstop Betting Sites
- Best Online Casino Canada
- Online Casinos Not On Gamstop
- オンラインカジノ 一覧
- Mejores Paginas De Poker
- Gambling Sites Not On Gamstop
- Casinos Not On Gamstop
- UK Casinos Not On Gamstop
- Non Gamstop Casino Sites UK
- Slots Not On Gamstop
- UK Online Casinos Not On Gamstop
- UK Casino Not On Gamstop
- Meilleur Casino En Ligne France
- Meilleur Casino En Ligne
- Casino Not On Gamstop
- Bookmaker Not On Gamstop
- Casino Non Aams
- Jeux Casino En Ligne
- Casino Online Esteri
- カジノ 仮想通貨
